Pv Nrt What Is N
| Ideal GASES AND THE Platonic GAS LAW This page looks at the assumptions which are made in the Kinetic Theory about ideal gases, and takes an introductory wait at the Ideal Gas Police force: pV = nRT. This is intended only as an introduction suitable for chemical science students at about UK A level standard (for 16 - eighteen year olds), and so in that location is no endeavour to derive the platonic gas law using physics-style calculations. Kinetic Theory assumptions about ideal gases There is no such matter as an ideal gas, of form, but many gases deport approximately equally if they were ideal at ordinary working temperatures and pressures. Real gases are dealt with in more detail on another folio. The assumptions are:
And so ii absolutely key assumptions, because these are the 2 about important ways in which real gases differ from ideal gases:
The Platonic Gas Equation The platonic gas equation is: pV = nRT On the whole, this is an easy equation to call up and use. The problems lie almost entirely in the units. I am bold below that you are working in strict SI units (equally you will exist if you are doing a UK-based exam, for example). Exploring the various terms Force per unit area, p Pressure is measured in pascals, Pa - sometimes expressed as newtons per square metre, N m-ii. These mean exactly the same thing. Exist conscientious if you lot are given pressures in kPa (kilopascals). For example, 150 kPa is 150000 Pa. You must make that conversion earlier y'all use the platonic gas equation. Should y'all want to convert from other pressure level measurements:
Volume, V This is the most probable place for you to go wrong when y'all use this equation. That'due south considering the SI unit of volume is the cubic metre, g3 - not cm3 or dm3. 1 m3 = yard dm3 = one 000 000 cm3 So if you are inserting values of volume into the equation, you lot start take to convert them into cubic metres. Yous would have to dissever a volume in dmiii by grand, or in cmthree past a meg. Similarly, if y'all are working out a volume using the equation, remember to covert the answer in cubic metres into dm3 or cm3 if y'all need to - this time by multiplying by a 1000 or a million. If yous get this wrong, you lot are going to end upward with a lightheaded answer, out by a factor of a g or a million. Then it is usually fairly obvious if you accept done something wrong, and you lot can check back again. Number of moles, due north This is easy, of course - information technology is but a number. You lot already know that you work information technology out by dividing the mass in grams by the mass of one mole in grams. You will near often utilise the ideal gas equation by outset making the substitution to give: I don't recommend that you recall the platonic gas equation in this form, but yous must be confident that you can convert it into this form. The gas constant, R A value for R will be given you if you demand information technology, or you lot can look information technology upwardly in a data source. The SI value for R is 8.31441 J K-i mol-1. | |
| Note:You may come up beyond other values for this with different units. A ordinarily used ane in the past was 82.053 cm3 atm 1000-i mol-i. The units tell you that the volume would be in cubic centimetres and the pressure in atmospheres. Unfortunately the units in the SI version aren't so evidently helpful. | |
| The temperature, T The temperature has to be in kelvin. Don't forget to add together 273 if yous are given a temperature in degrees Celsius. Using the platonic gas equation Calculations using the ideal gas equation are included in my calculations book (meet the link at the very bottom of the page), and I can't echo them here. There are, however, a couple of calculations that I haven't done in the book which requite a reasonable idea of how the ideal gas equation works. The molar volume at stp If you lot take done simple calculations from equations, you take probably used the molar volume of a gas. 1 mole of whatsoever gas occupies 22.4 dmiii at stp (standard temperature and force per unit area, taken as 0°C and i temper pressure). You may as well have used a value of 24.0 dmthree at room temperature and pressure level (taken as about twenty°C and 1 atmosphere). These figures are actually only true for an ideal gas, and we'll have a wait at where they come from. We tin can employ the ideal gas equation to calculate the volume of one mole of an platonic gas at 0°C and one atmosphere force per unit area. Offset, we have to get the units correct. 0°C is 273 K. T = 273 K 1 atmosphere = 101325 Pa. p = 101325 Pa We know that n = ane, because we are trying to summate the volume of ane mole of gas. And, finally, R = viii.31441 J K-1 mol-1. Slotting all of this into the ideal gas equation and then rearranging it gives: And finally, because we are interested in the volume in cubic decimetres, you have to remember to multiply this by chiliad to convert from cubic metres into cubic decimetres. The molar volume of an platonic gas is therefore 22.iv dmthree at stp. And, of form, you lot could redo this calculation to observe the book of ane mole of an ideal gas at room temperature and pressure - or any other temperature and pressure. Finding the relative formula mass of a gas from its density This is about as tricky as it gets using the ideal gas equation. The density of ethane is 1.264 g dm-iii at 20°C and 1 atmosphere. Calculate the relative formula mass of ethane. The density value ways that 1 dm3 of ethane weighs i.264 one thousand. Once again, before nosotros do anything else, get the awkward units sorted out. A force per unit area of 1 atmosphere is 101325 Pa. The book of 1 dm3 has to be converted to cubic metres, past dividing by one thousand. We have a book of 0.001 mthree. The temperature is 293 K. At present put all the numbers into the course of the platonic gas equation which lets you work with masses, and rearrange it to work out the mass of 1 mole. The mass of ane mole of anything is simply the relative formula mass in grams. Then the relative formula mass of ethane is 30.iv, to three sig figs. Now, if you lot add upward the relative formula mass of ethane, C2H6 using accurate values of relative atomic masses, you get an answer of 30.07 to iv pregnant figures. Which is different from our answer - so what'southward wrong? In that location are two possibilities.
If you need to know about real gases, now is a expert time to read well-nigh them.
Questions to examination your agreement If this is the outset set of questions you accept done, please read the introductory page before you start. You lot volition demand to use the Dorsum Push on your browser to come back here afterwards. questions on ideal gases answers
To explore real gases . . . To the kinetic theory menu . . . To the Physical Chemistry menu . . . To Main Menu . . . © Jim Clark 2010 (last modified July 2017) | |
Pv Nrt What Is N,
Source: https://www.chemguide.co.uk/physical/kt/idealgases.html
Posted by: stilesthicaltat.blogspot.com

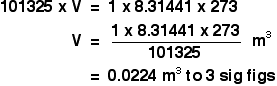
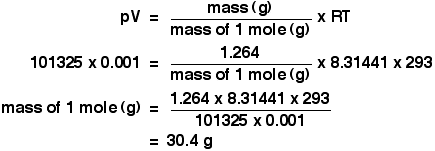


0 Response to "Pv Nrt What Is N"
Post a Comment